New Food Contact Substance Notification
1. Background
In accordance to Food Safety Law, companies who plan to use new substances (including new food contact resins and new food contact additives) in food contact materials or products on Chinese market must apply for and obtain a hygiene license from Health Administrative Department of the State Council.To meet the demand of industry, to ensure the safety of new food contact material, and to standardize new food contact material application procedure, the Ministry of Health (MOH) (now is NHFPC) hasdrawn up the‘Administrative Permission Regulation for New Food Related Products’ which specifically stipulated the concept, application, acceptance, administration permission of new food related products.
2. Our services
Initially set up by China Inspection and Quarantine (CIQ) Bureau in 2007 to provide REACH compliance services to Chinese chemical industry, CIRS has grown to be a leading provider of comprehensive chemical compliance services for companies doing businesses in/with China.
CIRS depends on rich chemical registration experience and technique,good communication with the authority to customize the notification plan for new food related products.To promote thecore competitiveness of enterprise,CIRS also provides one-stop solutions to your food related product regulatory issues in China.
1. Regulation consulting service, technical support
2. Invite relevant expert to analysis the product notification feasibility
3. Compile the notification dossier
4. Foreign documents translation, correction and notarization
5. Arrange tests and follow up communication in Chinese lab
6. Notification dossier assessment, examination, integration and submission.
7. Project progress tracking, and report the important progress in time
8. Attend the expertappraisalconference andassist enterprise answer the technical problem
9. Correct and improve the notification documents according to the appraisal committee suggestions
3. Notification dossier
3.1 Required documents
| Document name | Required documents |
| Application form | Chemical name, common name, chemical construction, molecular formula, molecular weight, CAS Number |
| physicochemical property | Melting point, boiling point, decomposition temperature, solubleness, chemical equations for potential side reactions occurred during manufacture or reaction with food |
| Technical necessity&Use condition |
1.Intended use, intended technical effect,application range, maximum dosage, minimum dosage for intended technical effect 2. Use condition:intended contact food category(Water- based food,oil food,acid food,alcohol food),contacttime and temperature, repeatable or not , the contact area/volume ratio of the contact material and food. |
| Manufacturing technique | Raw material and accessory material, process flow diagram, and technical parameters for each technical process. |
| Qualification specification requirements | Include purity, impurity ingredient, content and corresponding testing method, testing report. |
| Toxicology safety assessment documents |
1. Apply for new food contact material and food contact material additive shall provide toxicology safety assessment documents according to volume of migration. 2. Apply for the new ingredient of food use detergent and disinfectant shall provide toxicology safety assessment documents according to the food toxicology assessment analysis process and method (GB/T15193). 3. The toxicology assessment document shall be issued by GLP (good laboratory practice) laboratory. |
| Migration Testing &Analytical Methods&Consumer exposure |
1. According to theproduct use intention and use condition provide the migration date information, testing method and testing report. 2. The residual quantity date information for the product or conversion from the product in the food contact or packaging material, the corresponding testing method and testing report. 3. Consumer exposure and the assessment method information. 4. The testing report shall be issued by qualified laboratory |
| Documentary evidence for domestic and overseas approval | Approved documents which were issued by aboard governmental agencies, Industry Association or international organization |
| Other helpful documents | For example:exporting country relevant department or agent issue the documentary evidence for the product is allowed to produce or sale in domestic country |
| Power of attorney | Thepower of attorney shall be provided for the entrusted application |
3.2 Requiredtoxicology test item
| Migration quantity ( mg/kg) | Required toxicology test items | ||||
| QSAR Read-across and literature data | Three mutagenicity test | 90d Oral subchronic toxicitystudy | Reproductive toxicity |
Chronic toxicity and carcinogenicity |
|
| ≤0.01 | P | - | - | - | - |
| 0.01 -0.05 | P | P | - | - | - |
| 0.05-5.0 | P | P | P | - | - |
| 5.0-60 | P | P | P | P | P |
| High-molecular polymerb | P | - | - | - | - |
a. The three mutagenicity tests include: Ames test, Bone marrow cell micronucleus test and vitro mammalian cell chromosome aberration test.
b. For the high-molecular polymer(average molecular weight above 1000Dalton) shall provide each monomer toxicology safety assessment documents.
4. Service procedure
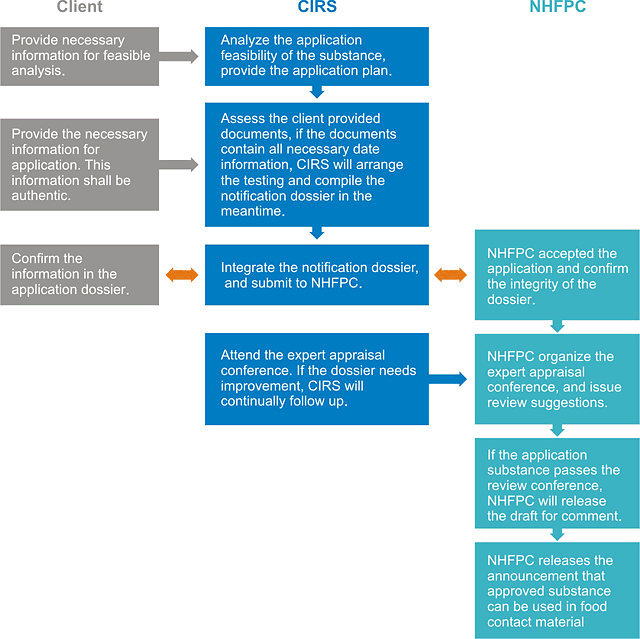
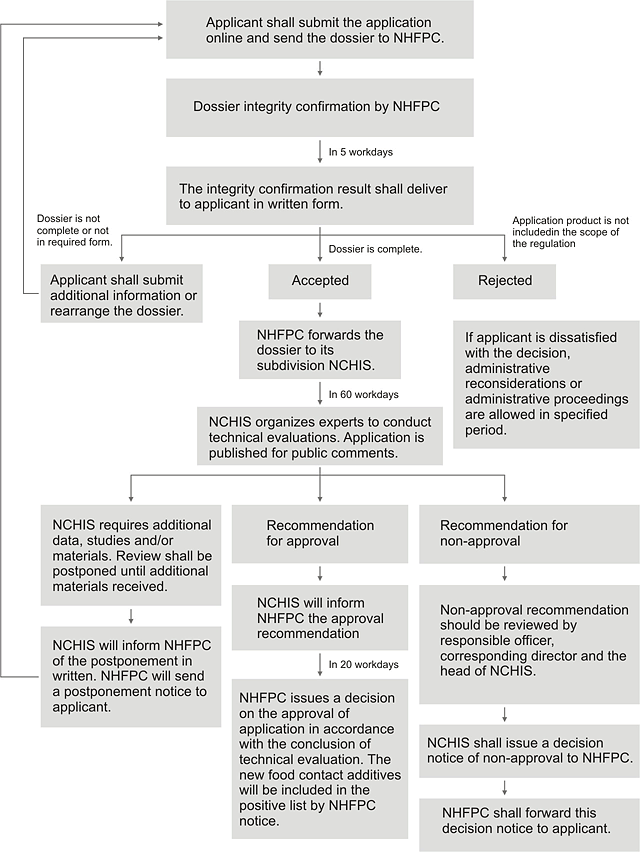
5. Work process of administrative approval
NHFPC: The National Health and Family Planning Commission
NCHIS: The National Center of Health Inspection and Supervision
Contact us
Ms. Cathy Yu Team Leader of Food Safety and Regulatory Affairs Department, CIRS China
11F Dongguan Building, 288 Qiuyi Road, Binjiang District, Hangzhou, China, 310020
Tel: +86 571 8720 6538 | Fax: +86 571 8720 6533
Email: cathy.yu@hfoushi.com






