Food labels in the US must comply with the requirements of American federal regulations – 21 CFR 101.9 for general food and 21 CFR 101.36 for dietary supplements. To convey the correct nutrition facts to consumers, label makers should fully understand the requirements of food labels and ensure that every detail is standardized. In this article, we will provide a detailed interpretation of the requirements of US nutrition labels.
Regular Food
- The basic format of nutrition facts
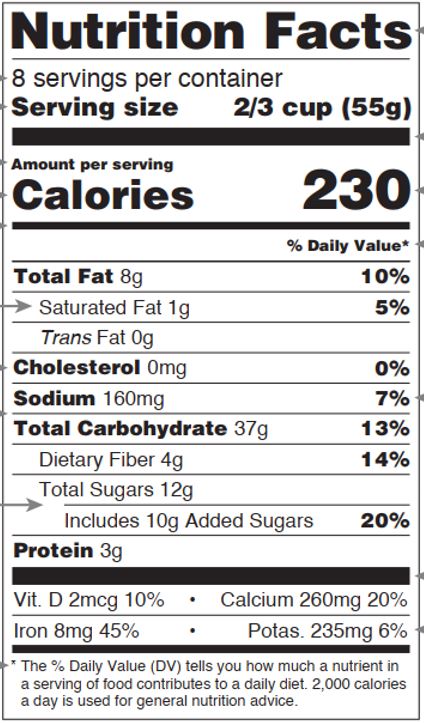
Fig.1 Basic format of nutrition facts (Appendix B to Part 101, Title 21)
- Label
The labels must be read as “Nutrition Facts”.
- Calorie calculation
The content of energy-yielding nutrients in each serving (before modification) was multiplied by the respective corresponding calorie conversion factors (see Table 1) and summed, and the numerical results were labeled according to the modification rules in Table 2. (21 CFR 101.9(c)(1)(i))
Table 1: Calorie conversion factors of energy-yielding nutrients in each serving
|
Nutrient |
Conversion factors calories/g |
Nutrient |
Conversion factors calories/g |
Nutrient |
Conversion factors calories/g |
|
Protein |
4 |
Isomaltitol |
2 |
Sorbitol |
2.6 |
|
Carbohydrates |
4 |
Lactitol |
2 |
Hydrogenated starch hydrolysate |
3 |
|
Fats |
9 |
Xylitol |
2.4 |
Mannitol |
1.6 |
|
Dietary Fiber |
2 |
Maltitol |
2.1 |
Erythritol |
0 |
- Nutrient details
The Nutrition Facts must label the 15 mandatory items in Table 2 in the order in which they are listed. Other vitamins and minerals are labeled voluntarily but are required to be labeled when they are added as a nutrient-enhancing additive or when they are the subject of a content claim (21 CFR 101.9(c)(8)(ii)). The order of labeling for other vitamins and minerals is shown in Table 3, and the order and modification rules for mandatory items is shown in the table below.
Table 2: Order and modification rules for mandatory items
|
Nutrient |
Label modification rules |
Regulations |
|
Calories |
<5 cal - expressed as 0 5-50 cal - Expressed to the nearest 5 cal increment >50 cal - Expressed to the nearest 10 cal increment |
21 CFR 101.9(c)(1) |
|
Total Fat |
<0.5 g - expressed as 0 g 0.5-5 g - Expressed to the nearest 0.5 g increment >5 g - expressed to the nearest 1 g increment |
21 CFR 101.9(c)(2) |
|
Saturated Fat |
||
|
Trans Fat |
||
|
Cholesterol |
<2 mg - expressed as 0 mg 2-5 mg - Expressed to <5 mg >5 mg - expressed to the nearest 5 mg increment |
21 CFR 101.9(c)(3) |
|
Sodium |
<5 mg - expressed to 0 mg 5-140 mg - expressed to the nearest 5 mg increment >140 mg - expressed to the nearest 10 mg increment |
21 CFR 101.9(c)(4) |
|
Total Carbohydrate |
<0.5 g - expressed as 0 g 0.5-1 g - expressed to <1 g >1 g - expressed to the nearest 1 g increment |
21 CFR 101.9(c)(6) |
|
Dietary Fiber |
||
|
Total Sugar |
||
|
Includes X g Added Sugars |
||
|
Protein |
21 CFR 101.9(c)(7) |
|
|
Vitamin D |
<2% RDI - %DV expressed as 0 2-10% RDI - %DV expressed to the nearest 2% increment 10-50% RDI - %DV expressed to the nearest 5% increment >50% RDI - %DV expressed to the nearest 10% increment |
21 CFR 101.9(c)(8) |
|
Calcium |
||
|
Iron |
||
|
Potassium |
- %DV calculation and its footnote
%Daily Value, referred to as %DV, is the percentage of labeled value (or value before modification) of each serving to daily nutrient reference values (DRVs/RDI) for that nutrient, expressed to the nearest 1 percent increment (21 CFR 101.9(d)(7)(ii)), except for vitamins-minerals (the modification rules for vitamins-minerals (excluding sodium) are the same as those for vitamin D in Table 2).
DRVs/RDI for adults and children aged 4 and above are listed in table 3.
Table 3 DRVs/RDI reference values(adults and children aged 4 and above as an example)
|
RDI |
|||
|
Vitamin D |
20 mcg |
Biotin |
30 mcg |
|
Calcium |
1,300 mg |
Pantothenic acid |
5 mg |
|
Iron |
18 mg |
Phosphorus |
1,250 mg |
|
Potassium |
4,700 mg |
Iodine |
150 mcg |
|
Vitamin A |
900 mcg |
Magnesium |
420 mg |
|
Vitamin C |
90 mg |
Zinc |
11 mg |
|
Vitamin E |
15 mg |
Selenium |
55 mcg |
|
Vitamin K |
120 mcg |
Copper |
0.9 mg |
|
Thiamin |
1.2 mg |
Manganese |
2.3 mg |
|
Riboflavin |
1.3 mg |
Chromium |
35 mcg |
|
Niacin |
16 mg |
Molybdenum |
45 mcg |
|
Vitamin B6 |
1.7 mg |
Chloride |
2,300 mg |
|
Folate |
400 mcg DFE |
Choline |
550 mg |
|
Vitamin B12 |
2.4 mcg |
Protein |
/ |
|
DRVs |
|||
|
Fat |
78 g |
Sodium |
2,300 mg |
|
Saturated Fat |
20 g |
Dietary Fiber |
28 g |
|
Cholesterol |
300 mg |
Protein |
50 g |
|
Total carbohydrate |
275 g |
Added Sugars |
50 g |
Note: DFE = Dietary Folate Equivalents; 1 mcg DFE=0.6 mcg folic acid.
No DRVs/RDIs for trans fats, or total sugars, there is no need to list %DV. If the product is intended for use by the general population 4 years of age and older and there is no claim on the label for protein content (e.g., high protein content), there is no need to list the %DV for protein.
%Daily Value* footnote: *The % Daily Value tells you how much a nutrient in a serving of food contributes to a daily diet. 2,000 (or 1,000, depending on the population) calories a day is used for general nutrition advice.(21 CFR 101.9(d)(9))
Dietary Supplement
- The basic format of supplement facts
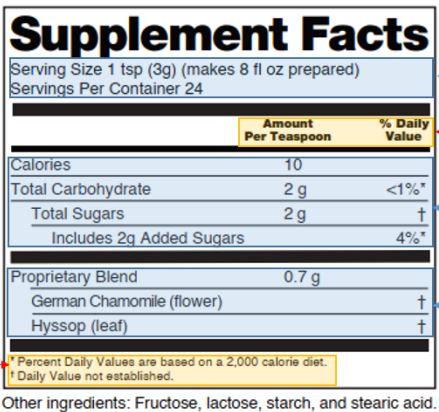
Fig. 2 Basic format of supplement facts
- Title
The title must be read as “Supplement Facts”.
- Calorie calculation
Calorie calculation in the Supplement Facts is the same as that for regular food but does not need to be conspicuously labeled. When calories per serving are <5cal, calories do not need to be labeled in the Supplement Facts.
- Dietary supplement details
(1) Regular ingredients
The order must be listed as the following: calories, total fat, saturated fat, trans fat, cholesterol, total carbohydrates, dietary fiber, total sugars, added sugars, protein, vitamin A, vitamin C, vitamin D, vitamin E, vitamin K, thiamine, riboflavin, niacin, vitamin B6, folate, vitamin B12, biotin, pantothenic acid, choline, calcium, iron, phosphorus, iodine, magnesium, Zinc, Selenium, Copper, Manganese, Chromium, Molybdenum, Chloride, Sodium, Potassium, and Fluoride (21 CFR 101.36(b)(2)). The above ingredients that are not present or are expressed as zero according to the modification rules for the Nutrition Facts (see Table 2) are not labeled in the Supplement Facts.
(2) Other ingredients
Probiotics, curcumin, must be listed below and separated from those in (1) by a bold horizontal line. If it is a botanical ingredient, the Latin name and part of the source must be listed. (21 CFR 101.4(h))
- %DV calculation and its footnote
(1) When calculating %DV, total fat, saturated fat, cholesterol, sodium, potassium, total carbohydrates, and dietary fiber may be calculated using the values on the supplement facts, and all other ingredients must be calculated using the values before modification. (21 CFR 101.36(b)(2)(iii)(B))
(2) The %DV is expressed to the nearest 1% increment. When the amount of a dietary ingredient is so small (but above the threshold value of 0) that the %DV would be zero when rounded, the %DV should be declared by using the terms “less than 1%” or “<1%” (e.g., a product containing 1 g of total carbohydrates would list %DV as “less than 1%” or “<1%”). (21 CFR 101.36(b)(2)(iii)(C))
(3) If total fat, saturated fat, total carbohydrates, dietary fiber, protein, or added sugars are indicated, the %DV values for these ingredients are marked with a specific symbol in conjunction with the footnote “Percent Daily Values are based on a 2,000 (or 1,000) calorie diet. “ (21 CFR 101.36(b)(2)(iii)(D))
(4) If a dietary ingredient without RDIs/DRVs is indicated, it is described in a footnote “Daily Value not established”. (21 CFR 101.36(b)(2)(iii)(F))
Conclusion
The format of the label is not limited to the given sample, it can be adjusted according to the size of the food package and the different contents, as well as the font size and line thickness. The details need to be further referred to the regulations. If you have any questions or need any help about US food labeling, please feel free to contact us at service@hfoushi.com.

