It’s stipulated in Article 8 of the Regulations on the Administration of Feeds and Feed Additives that, before newly developed feeds or feed additives are put into production, the developer or production enterprise must submit an approval application to the competent agricultural authority of the State Council, and provide the following samples and documentation for the new feeds or feed additives:
- Detailed information including the name, primary components, physicochemical properties, development methodology, production processes, quality standards, test methods, test reports, stability test reports, environmental impact assessments, and pollution prevention measures;
- Reports on feeding effectiveness, residual dissolution dynamics, and toxicological safety evaluations for the new feeds or feed additives issued by testing agencies designated by the competent agricultural authority of the State Council; and
- For applications seeking approval for new feed additives, it is also required to specify the purpose and methods of use for the new feed additive and provide an analytical evaluation report on the potential impact of its residues on human health.
Relevant Laws and Regulations
- Regulations on the Administration of Feeds and Feed Additives
- Administrative Measures for New Feeds and New Feed Additives
- Application Dossiers Requirements for New Feeds and New Feed Additives
Application Scope
- Newly developed single feeds and feed additives that have not yet been approved for use within China;
- Feed additives with an expanded scope of application;
- Feed additives with content specifications lower than the requirements for safe use of feed additives, except for those prepared by mixing feed additives with carriers or diluents in certain proportions;
- Feed additives with significant changes in the production process;
- New feeds or new feed additives that have not been put into production for more than 3 years since obtaining the certificate, and are applied for production by other enterprises; and
- Other situations specified by the Ministry of Agriculture.
Qualification Requirements for Applicants
The developers or production enterprises of new feeds and new feed additives.
Applicant Protection Period
For new feeds and new feed additives, there is a monitoring period of 5 years, starting from the date of issuance of the certificate. During this period, no other production or import registration applications for the same new feeds or feed additives will be accepted, except for cases where the new feeds or feed additives have not been put into production for more than 3 years.
Competent Authorities
- Accepting agency: Ministry of Agriculture and Rural Affairs of the People’s Republic of China (MARA) Government Affairs Service Hall
- Review agency: National Feed Review Committee (hereinafter referred to as the Review Committee)
- Decision-making agency: Bureau of Animal Husbandry and Veterinary Medicine, MARA
Application Dossiers and Samples Required
|
Application dossiers requirements |
|
|
1 |
Application Form for the Approval of New Feeds and New Feed Additives |
|
2 |
Product name, naming basis, category, and purpose of product development |
|
3 |
Identification report of active ingredients, chemical structure, and physicochemical properties, or report on the classification identification of animals, plants, and microorganisms |
|
4 |
Product functions, application scope, usage methods, recommended dosage in mixed feed or complete mixed rations, and, if necessary, provide maximum levels |
|
5 |
Production process, manufacturing method, and product stability test report (the test report should be stamped with the official seal of the issuing unit, and signed by the responsible person and the testing personnel) |
|
6 |
Draft quality standards and compilation instructions, and product test reports. If there are maximum level requirements, test methods for active ingredients in mixed feed, concentrated feed, feed supplements, and premixes should also be provided (the testing report should be stamped with the official seal of the issuing unit and signed by the responsible person and the testing personnel). |
|
7 |
Product efficacy evaluation test report, safety evaluation test report, including target animal tolerance assessment, toxicological safety assessment, metabolism, and residue evaluation, among others (the test report should be stamped with the official seal of the issuing unit and signed by the responsible person and the testing personnel); For applications seeking approval for new feed additives, an analytical evaluation report on the potential impact of residues of the new feed additive on human health in animal products should also be provided. |
|
8 |
Label design, packaging requirements, storage conditions, shelf life, and precautions |
|
9 |
Summary of pilot production and report on the treatment of three wastes (waste gas, wastewater, and waste residue) |
|
10 |
For products submitted jointly, a joint application agreement is required. For genetically modified products, a copy of the approval document issued by MARA should be provided. |
|
Sample requirements |
|
|
1 |
Originating from pilot or industrial production lines |
|
2 |
Three consecutive batches of samples should be provided for each product, with four samples from each batch. Each sample should be at least five times the amount required for testing. |
|
3 |
If necessary, provide relevant standard or chemical references. |
Application Process and Timeline
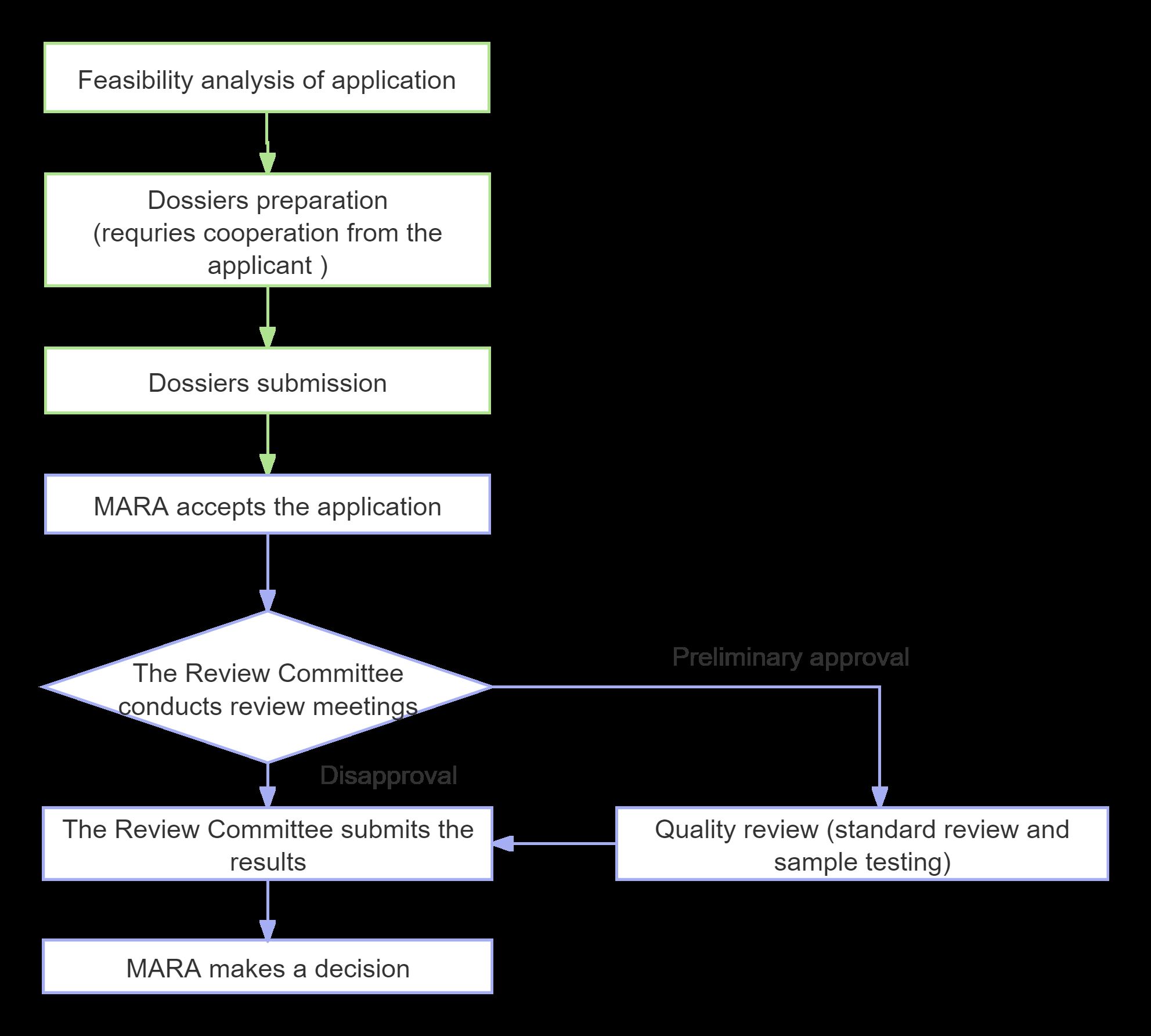
Note: The entire application cycle, from dossiers preparation and experimentation to the issuance of certificates for the new feed and feed additives, typically spans 2 to 3 years.