On March 13, 2024, the National Health Commission of the People’s Republic of China (NHC) issued an announcement (No. 2 of 2024) on the approval of 23 “Three New Foods”, including 6 new food raw materials, 12 new food additives, and 5 new food-related products. Details are as follows:
New food raw materials (6 types)
Dendrobium protocorm
|
Name |
Dendrobium protocorm |
|
|
Basic information |
Source: Dendrobium officinale Kimura et Migo or Dendrobium huoshanense C. Z. Tang et S. J. Cheng |
|
|
Brief introduction of the production process |
Protocorms are obtained through tissue culture using the seeds or stem of Dendrobium officinale Kimura et Migo or Dendrobium huoshanense C. Z. Tang et S. J. Cheng as raw materials, followed by collection, drying and other processes. |
|
|
Recommended intake |
Dehydrated products ≤ 3.5g/day |
|
|
Other information |
1. The labels and instructions shall bear a statement indicating that they should not be consumed by infants, pregnant women and lactating women, along with the recommended intake. 2. Food safety indicators must meet the following requirements: |
|
|
Pb, mg/kg ≤ |
1.0 |
|
|
Hg, mg/kg ≤ |
0.1 |
|
|
As, mg/kg ≤ |
0.3 |
|
|
Colony count, CFU/g ≤ |
30000 |
|
|
Coliforms, MPN/g ≤ |
0.92 |
|
|
Mould and Yeast, CFU/g ≤ |
50 |
|
|
Salmonella, /25g |
0 |
|
|
Staphylococcus aureus, /25g |
0 |
|
|
Plant growth regulator, mg/kg≤ |
0.05 |
|
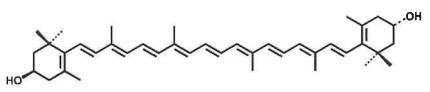
Meso-zeaxanthin
|
Name |
Meso-zeaxanthin |
||
|
Basic information |
Source: Tagetes erecta L. Structure:
CAS number: 31272-50-1 Molecular Formula: C40H56O2 Relative molecular mass: 568.88 |
||
|
Brief introduction of the production process |
Prepared through dehydration, grinding, extraction, isomerization, purification, and other processes using flowers of natural Tagetes erecta L. as the raw material. |
||
|
Recommended intake |
≤ 8 mg/day (count as Meso-zeaxanthin) |
||
|
Quality requirements |
Property |
Reddish orange powder |
|
|
Meso-zeaxanthin, g/100g ≥ |
54.0 (See Appendix A for the test methods) |
||
|
Moisture, g/100 g ≤ |
5.0 |
||
|
Other information |
1. The applicable scope of use does not include infants and young children food. 2. The detection method for n-Hexane residue shall follow GB 24405. 3. Food safety indicators must comply with the following requirements: |
||
|
n-Hexane, mg/kg ≤ |
10.0 |
||
|
Pb, mg/kg ≤ |
1.0 |
||
|
Cd, mg/kg ≤ |
0.5 |
||
|
Hg, mg/kg ≤ |
0.1 |
||
|
As, mg/kg ≤ |
1.0 |
||
|
benzo[a]pyrene, μg/kg ≤ |
2.0 |
||
|
Colony count, CFU/g ≤ |
1000 |
||
|
Coliforms, CFU/g ≤ |
10 |
||
|
Mould, CFU/g ≤ |
100 |
||
|
Yeast, CFU/g ≤ |
100 |
||
|
Salmonella, /25g |
0 |
||
|
Staphylococcus aureus, /25g |
0 |
||
|
Listeria monocytogenes, /25g |
0 |
||
Pichia kluyveri
|
Name |
Pichia kluyveri |
|
Other information |
1. Being included in the List of Strains that Can be Used in Food, and approved for use in fermentation processing of fermented wine, fruit and vegetable juices and drinks, tea drinks and botanical drinks, excluding infants and young children food. The scope of use shall be indicated on the labels and instructions. 2. Food safety indicators shall comply with the provisions of GB 31639-2023 National Food Safety Standard - Food Processing Strains Preparation. |
Bacillus subtilis DE111
|
Name |
Bacillus subtilis DE111 |
|
Other information |
1. Being included in the List of Strains that Can be Used in Food. The applicable scope of use does not include infants and young children food. 2. Food safety indicators shall comply with the provisions of GB 31639-2023 National Food Safety Standard - Food Processing Strains Preparation. |
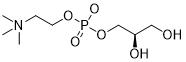
L-alpha-Glycerylphosphorylcholine
|
Name |
L-alpha-Glycerylphosphorylcholine |
|
Basic information |
Structure:
CAS number: 28319-77-9 Molecular formula: C8H20NO6P Relative molecular mass: 257.22 |
|
Brief introduction of the production process |
Prepared through condensation and esterification reactions, followed by decolorization, impurity removal, concentration, refinement, drying and other processes using polyphosphoric acid, choline chloride, R-3-Chloro-1,2-propanediol, sodium hydroxide and water as raw materials. |
|
Recommended intake |
≤ 600 mg/day (on a dry basis) |
|
Other information |
1. The labels and instructions shall bear a statement indicating that they should not be consumed by infants, pregnant women and lactating women, along with the unsuitable group and recommended intake. 2. See the Appendix for quality specifications and food safety indicators. |
Leuconostoc pseudomesenteroides
|
Name |
Leuconostoc pseudomesenteroides |
|
Other information |
1. Being included in the List of Strains that Can be Used in Food, and approved for use in fermented milk, flavored fermented milk, cheese, fermented milk drinks, lactobacillus drinks (non-solid beverages), cream, butter and anhydrous milkfat, excluding infants and young children food. 2. Food safety indicators shall comply with the provisions of GB 31639-2023 National Food Safety Standard - Food Processing Strains Preparation. |
New food additives (12 types)
New food additives (2 types)
|
S.N. |
Name |
Function |
Category number |
Food name/category |
Maximum level (g/kg) |
Remark |
|
1 |
Mixed tocotrienol tocopherol concentrate |
Antioxidant |
02.01.01 |
Plant oils and fats |
0.2 |
Counted as the total amount of tocopherol and tocotrienol |
|
2 |
Enzymatically produced steviol glycosides |
Sweetener |
01.01.03 |
Modified milk |
0.18 |
It can be used alone or in combination with steviol glycosides, and count as steviol equivalents. |
|
01.02.02 |
Flavored fermented milk |
0.2 |
||||
|
03.01 |
Ice cream and ice milk |
0.5 |
||||
|
05.02.01 |
Gum-based candy |
3.5 |
||||
|
14.0 |
Beverages, excluding 14.01 packaged drinking water; 14.02.01 fruit and vegetable juices (pulp); 14.02.02 concentrated fruit and vegetable juice (pulp) |
0.2 |
It can be used alone or in combination with steviol glycosides, and count as steviol equivalents. Count as the state of ready-to-drink, increase the usage of solid drinks according to the dilution ratio. |
Table A.1 Information on the production strain used for enzymatically produced steviol glycosides
|
Food additive |
Source |
Donor |
|
Enzymatically produced steviol glycosides |
Escherichia coli BL21 (DE3) |
Methylocaldum Szegediense a , Stevia rebaudiana Bertoni b and Solanum tuberosum c |
a refers to the donor of sucrose synthase
b refers to the donor of β-1,3-glycosyltransferase
c refers to the donor of β-1,2-glycosyltransferase
New food enzymes (3 types)
|
S.N. |
Enzyme |
Source |
Donor |
|
1 |
D-psicose 3-epimerase |
Bacillus subtilis |
Clostridium scindens ATCC35704 |
|
2 |
Cyclomaltodextin glucanotransferase |
Anoxybacillus caldiproteolyticus |
— |
|
3 |
Cellulase |
Penicillium oxalicum |
— |
*The quality specifications of the enzymes shall meet the provisions specified in GB 1886.174 National Food Safety Standard - Food Additives, Enzymes.
New food nutrition enhancers (2 types)
- 2’-fucosyllactose,2’-FL
The maximum levels, the scope of use and quality specifications shall meet the requirements outlined in NHC Notice No. 8 of 2023, excluding Appendix C. The production strain information is given in the Table below:
Table 1 Information on the production strain of 2’-fucosyllactose
|
Nutrition enhancer |
Source |
Donor |
|
2’-fucosyllactose |
Escherichia coli BL21(DE3) |
Helicobacter pylori a |
a refers to the donor of α-1, 2-fucosyltransferase
- d-Ribose
|
Nutrition enhancer |
Category number |
Food name/category |
Maximum level |
Remark |
|
d-Ribose |
13.05 |
Foods for special dietary purposes excluding 13.01-13.04 (sports nutrition foods only) |
1-2g/day |
— |
Food additives with expanded scope and usage levels (5 types)
|
S.N. |
Name |
Function |
Category number |
Food name/category |
Maximum level (g/kg) |
Remark |
|
1 |
Propyleneglycol alginate |
Thickener |
06.05.02.01 |
Bean vermicelli, bean noodles |
1.5 |
— |
|
06.05.02.04 |
Round rice ball |
|||||
|
2 |
Polyoxyethylene (20) sorbitan monooleat |
Emulsifier |
16.03 |
Collagen casing |
0.5 |
— |
|
3 |
Ascorbyl palmitate (enzymatic) |
Antioxidant |
01.03.02 |
Formulated milk powder and formulated cream powder |
0.2 |
Count as the ascorbic acid in fat |
|
07.01 |
Bread |
0.2 |
— |
|||
|
14.05.01 |
Tea drinks |
0.2 |
Count as the state of ready-to-drink, increase the usage of solid drinks according to the dilution ratio. |
|||
|
4 |
Rosemary extract |
Antioxidant |
04.05.02 |
Processes nuts and seeds |
0.3 |
— |
|
5 |
Sucralose |
Sweetener |
04.05.02.01.01 |
Shelled and cooked processed nuts and seeds |
4.0 |
— |
|
04.05.02.01.02 |
Unshelled and cooked processed nuts and seeds |
2.0 |
New food-related products (5 types)
New food contact additives (2 types)
|
S.N. |
Name |
CAS number |
Scope of use |
Maximum level (%) |
|
1 |
Chromium iron oxide |
12737-27-8 |
Plastic |
2 |
|
2 |
Bicyclo[2.2.1] heptane-2,3-dicarboxylic acid, calcium salt (1:1), (1R,2R,3S,4S)-rel- |
839683-04-4 |
Plastic: PP; PE |
0.25 |
New food contact resins (2 types)
|
S.N. |
Name |
CAS number |
Scope of use |
Common category name |
Maximum level (%) |
|
1 |
Polybutylene succinate adipate |
67423-06-7 |
Plastic |
PBSA |
Appropriate level |
|
2 |
1,3-Benzenedicarboxylic acid, polymer with 1,4-benzenedicarboxylic acid and 1,4-cyclohexanedimethanol |
26124-27-6 |
Plastic |
PCT |
Appropriate level |
Food contact resins with expanded scope of use (1 type)
|
S.N. |
Name |
CAS number |
Scope of use |
Common category name |
Maximum level (%) |
|
1 |
Undecanoicacid,11-amino-,homopolymer |
25587-80-8 |
Plastic |
PA |
Appropriate level |
If you need any assistance or have any questions, please get in touch with us via service@hfoushi.com.
Further information
CFSA Ne ws