In November 2021, the Scientific Committee for Consumer Safety of the European Union (SCCS) issued a preliminary assessment draft on the cosmetic whitening ingredient Kojic acid, concluding that Kojic acid has potential endocrine-disrupting properties and a maximum concentration of 1% allowed for use in cosmetics in the European Union is no longer considered safe.
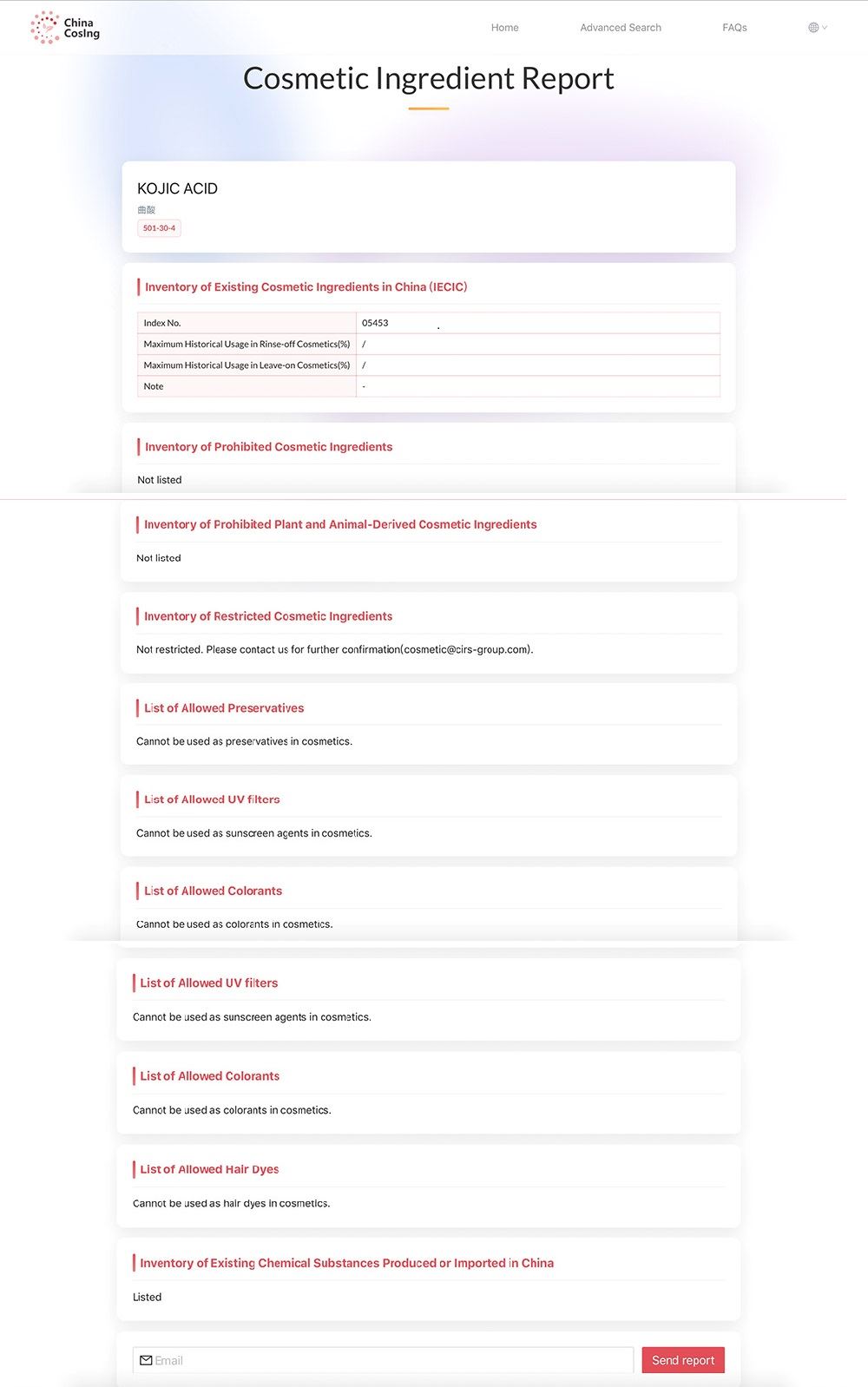
Kojic acid, CAS Number 501-30-4. Kojic acid is an active ingredient in whitening and anti-freckle cosmetics. It can inhibit the activity of tyrosinase in human body and control the pigmentation of skin. In the upsurge of brush acid of domestic cosmetics, koji acid can also be regarded as the main force.
The SCCS issued safety assessments of kojic acid in 2008 and 2012, respectively, indicating that it is safe in humans at a maximum concentration of 1% used in leave-in products. Kojic acid was added to the SCCS priority reevaluation list after new data indicated that kojic acid may have endocrine disrupting properties that interfere with thyroid function, such as preventing organic iodination or affecting iodine absorption.
The new assessment suggests that the maximum concentration of kojic acid in cosmetics at 1% is no longer safe, and that SCCS does not provide a maximum safe concentration for kojic acid in a single cosmetic. The use concentration of Kojic acid should not exceed 0.04% when used in combination with multiple cosmetics. Kojic acid derivatives were not evaluated in the opinion due to lack of data.
At present, the evaluation conclusion of kojic acid by CIR is that the maximum allowable concentration of kojic acid (1.0% by mass fraction) is safe under certain use conditions.
The query information of Chinacosing Database of Cosmetics Raw Materials independently developed by CIRS Group shows that kojic acid has been listed in the IECIC, and there is no restriction condition on Safety and Technical Standards for Cosmetics. If enterprises use this raw material in cosmetics, they need to carry out safety assessments to ensure its safety.