updated by David Wan on 25 March 2016

Ms. April Guo
Manager of Personal and Home Care Division, CIRS China
CIRS China
Email: service@hfoushi.com
Download this guidance in PDF form .
Current regulations
- Regulations concerning the hygiene supervision over cosmetics(1990);
- Detailed Rules for the Implementation of the Regulation on the Hygiene Supervision over Cosmetics(2005);
- Hygienic Standard for Cosmetics(2007)
- The Measures for the Administration of Hygiene License for Cosmetics (revised in 2010);
- Guideline for Risk Evaluation of Substances with Possibility of Safety Risk in Cosmetics(2010);
- Standard Chinese Names of International Cosmetics Ingredients Inventory (2010);
- Cosmetics Technical Requirement Standard(2011);
- AQSIQ Order No. 143 of 2011 - The Administrative Measures on the Inspection, Quarantine and Supervision of Chinese Imported & Exported Cosmetics (2011)
Regulation upcoming changes
- Technical Safety Standard for Cosmetics will be effective on 1st Dec 2016 to replace Hygienic Standard for Cosmetics (2007).
- Inventory of Existing Cosmetic Ingredients in China (IECIC 2015, Final Version)
- Guidelines on Safety Risk Assessment of Cosmetic Products (Draft, 2015)
- Regulations Concerning the Supervision and Administration of Cosmetics (Draft, 2015)
- Standards for Record-Keeping Management of Cross-border E-commerce Operators and Products available on 1st Jan 2016.
Definition of Cosmetics and Classifications
Cosmetics are defined as daily used industrial chemicals which can be spread on the outer surface of human body (e.g. skin, hairs, nails. lips etc) for the purpose of cleaning, deodorizing, providing skin care, beauty and make – up, by way of smearing, spraying or other similar means.
Note: new definition indraft Regulations Concerning the Supervision and Administration of Cosmeticsis the products which can be spread on the outer surface of human body (e.g. skin, hairs, nails. lips etc), the teeth and oral mucosa for the purpose of cleaning, protecting, beautifying, deodorizing and keeping in good condition, by way of smearing, spraying or other similar means.
Cosmetics are divided into two classes: non special use cosmetics and special use cosmetics. Each class requires different type of license from CFDA or FDA at provincial level.
|
Country of Origin |
Type of Cosmetics |
Official Approval Mode |
Who Shall Apply? |
Who Will Be the Competent Authority? |
|
Mainland China |
Non special use cosmetics hair care, nail care, skin care, perfumes and make-up |
Record keeping number issued on CFDA website |
Domestic manufacturer or brand owner |
FDA at provincial level |
|
Foreign countries |
Record keeping certificate |
A Chinese responsible person appointed by foreign manufacturer |
CFDA |
|
|
Mainland China |
Special use cosmetics products for hair growth, hair dye, hair perm, hair removal, breast shaping, fitness, deodorizing, spots removal(whitening) and sun block |
Hygiene license |
Domestic manufacturer or brand owner |
CFDA |
|
Foreign countries |
Hygiene license |
A Chinese responsible person appointed by foreign manufacturer |
CFDA |
Exemptions for the Application of Pre-market Approval License
All cosmetics have to be made record-keeping or approved with CFDA or local FDA at provincial level before they are placed in China market. However, it is not required for soaps, toothpaste and oral cleaner. They can be imported into China directly after custom clearance.
Registration Procedures of Imported Cosmetics
The diagram below is an overview of the CFDA registration process of cosmetics, step by step explained in the subsequent paragraphs.
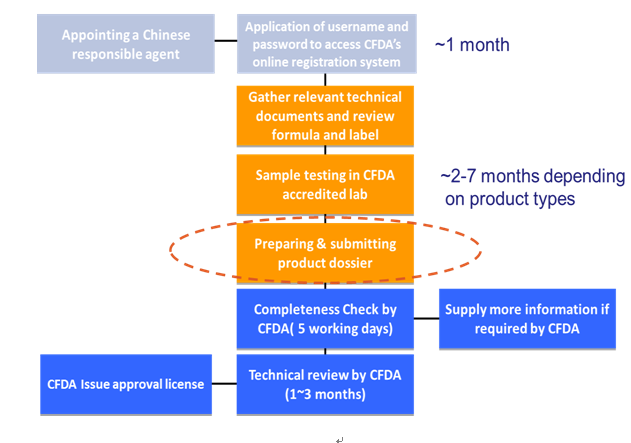
Note:It is suggested that both the English and Chinese brand name should be registerred in China before or during the registration of products. Once your brand name is registerred by another company, you could not use your brand name in China. It will be the huge losses for foreign manufacturers.
1. Appointing A Chinese Responsible Agent
A foreign company can’t register products directly and must appoint a Chinese responsible agent to apply for the CFDA license via a written Power of Attorney. The Chinese responsible agent is solely responsible for product registration. The agent does not undertake responsibility of product safety yet and its name does not need to appear on product labels.
A foreign manufacturer has only one responsible agent in China. But different foreign manufacturers can have the same responsible agent in China to apply for the CFDA license.
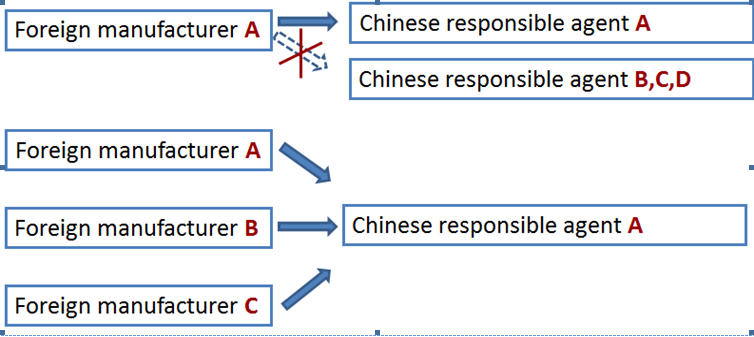
The legal entity either a Chinese subsidiary company, a distributor or a third party such as consulting firm can act as the responsible agent. Nevertheless, CIRS has summarized the differences regarding who act as the responsible agent.
1) When a Chinese subsidiary company acts as the responsible person,
Advantage:
- There is no risk for the owner of license.
- It is easier to solve the custom clearance when the foreign manufacturer has several distributors
Disadvantage:
- Requiring higher cost to establish new subsidiary in China.
- Product registration should be started after the registration of subsidiary is finished.
2) When a distributor acts as the responsible person,
Advantage:
- The distributor might help to handle the pre-market registration to save the cost for foreign manufacturer.
- Local advantage to look for an agent to address the pre-market registration
Disadvantage:
- If the distributor pay or share the cost of registration, it will be difficult for foreign manufacturer to own the approval license.
- Hard to change the distributor
- Confidential info such as detailed formulas, production process will be revealed because the responsible person has to seal all registration dossiers
3) When a third party such as consulting firm acts as the responsible person,
Advantage:
- The foreign company will be the holder of approval license definitely.
- Having rich experience to handle the cases more professionally and efficiently so as to save the time and cost.
Disadvantage:
- Consulting service is an outcome of economic development. The quality of consulting service is uneven in China so far. Even more there are no requirements for the qualification of responsible person except the legal entity in China, so it is difficult for foreign companies to find a satisfied consulting firm with high-qualified and professional service.
2.Application of User Name and Password
The Chinese responsible agent needs to apply for a username and password to access CFDA’s online registration system before registering cosmetic products. Notarized appointment letter and business license of the agent will be required.
3. Product Formula
Product formula should be provided in details including the INCI name, standard Chinese name, concentration and function of each ingredient. If the formula contains banned ingredients or does not meet the requirements of restrictive conditions in hygienic standard for cosmetics (2007)(will be replaced by Technical Safety Standard for Cosmetics on 1 Dec. 2016), the product will not be approved by CFDA. Moreover, if a product contains a new cosmetic ingredient, the new cosmetic ingredient will also need CFDA’s approval. Inventory of Existing Cosmetic Ingredients in China (IECIC, 2015)()is a reference to check the new cosmetic ingredient. It is advised that the product formula has to be reviewed before deciding to submit registrations to CFDA.
4. Original Product Package
The original product package contains product name, full ingredient list, claiming and company information of manufacturer. Claiming would be a main issue of package. According to Guidelines of Cosmetics Nomenclature, using absolute and exaggerated words and having medical effect are not allowed. For example, anti-bacterial, growth factor, stem cells, top-level, etc. Therefore, the product package has to be reviewed as well before deciding to submit registrations to CFDA.
5. Testing Requirements
Cosmetics need to be tested in CFDA-designated labs in China during the registration process even if they have been tested abroad or assessed. The CFDA has authorized 21 labs to perform hygiene safety tests and 6 labs to conduct human safety tests. Hygiene safety tests include physio-chemical, microbiological and toxicological studies, which are mandatory for non-special use cosmetics. For special use cosmetics, human safety tests are also required. If a cosmetic product contains risk substances, i.e., components (impurities or additives) that may cause potential harm to human health resulting from raw materials or materials brought in during the production process, then additional tests are required. So far, all toxicological tests are performed in animals in terms of OECD methods. As we know, animal testing can be waived for domestic non special use cosmetics from 30thJun 2014. For foreign companies claiming their products are cruelty free, there is a possible way for them to place their products in China without animal testing. They can export the bulk to China for filling and packing. Then the products manufactured under this way are regarded as domestic cosmetics. Currently, another way to avoid animal testing is selling cosmetics by cross-border e-commerce. The details about the management of online cosmetics in China can be found .
6. Registration Dossier
The list of documents below is required for the registration of imported ordinary cosmetics:
| Imported non special use cosmetics |
|
1) Application form for the cosmetic product to be imported |
|
2) Chinese product name and nomenclature |
|
3) Product formula |
|
4) Product quality and safety control file (The product info such as appearance, flavor, batch no and shelf life is required. Other quality control index like heavy metals and microbiology should be provided as well.) |
|
5) Original product packaging including labelling information and product information sheet |
|
6) Testing report and relevant data from inspection organization certified by CFDA |
|
7) Safety assessment report of cosmetics containing potential risk substances (Besides risk substances in formula, each ingredient might be assessed in terms of draft Guidelines on Safety Risk Assessment of Cosmetic Products in the future.) |
|
8) Stamped copies of power of attorney and business license of Chinese responsible agent; |
|
9) Statement from manufacturer guaranteeing that materials used meet the requirements of BSE free regions. |
|
10)Certificate of free sale at production country (region) or country (region) of origin |
|
11)Brief description and diagram of production process |
|
12)Technical requirements for cosmetic products in text |
|
13)Other relevant information which can support the application (For baby care products, principle of formula design and requirements for the selection of raw materials are needed.) |
Besides aforementioned documents, more documents are required for special use cosmetics.Efficacy ingredients and proof of efficacy for hair growth, fitness and breast shaping cosmetics are needed.
7. CFDA Review
CFDA will conduct a format review or completeness check first and then a technical review. The CFDA holds a meeting once a month to conduct the technical review of the registration of special use cosmetics. There is no fixed time to perform the technical review of registration of non-special use cosmetics. The format review would take five working days. The technical review time will be 3 months generally. If one application has been approved, a certificate will be issued by the CFDA. The certificate is also required for customs clearance. The certificate number must be marked/ displayed on the Chinese label.
8. Duration and Cost of Registration
The duration and cost of registration depends on the type of cosmetics. In general it takes 6-8 months to register imported non-special use cosmetics and 9-14months to register special use cosmetics. The registration cost consists of testing fee and consulting fee. Testing fee is charged by the CFDA designated labs. The CFDA does not charge any administration fee. The total fee for special use cosmetics is much higher than non-special use cosmetics because human safety tests are required in addition to hygiene safety tests. The estimated cost of registration of cosmetics is listed as follows:
|
Type of cosmetics |
Estimate cost (RMB per type)* |
|
Imported non-special use cosmetics |
12,000-18,000 |
|
Imported special use cosmetics |
19,000-80,000 |
* Extra tests need to be conducted if cosmetic products contain efficacy ingredients or potential risk substances.
9. Customs Clearance
After receiving CFDA approval license, foreign manufacturers are now qualified to export their cosmetics to China. When goods arrive at Chinese ports, importers need to apply for an inspection from the local branch of the China Inspection and Quarantine Bureau (port CIQ). The importer needs to show the hard copy of CFDA license and submit the copy of the license, product formula, a compliance declaration, sample page of Chinese label, original label and translation version to port CIQ prior to inspections. The port CIQ will do a random sample check and conduct Physio-chemical and microbiological tests. The port CIQ will also review the Chinese label according to GB 5296.3-2008. If the goods pass CIQ’s inspections, the port CIQ will issue a certificate of inspection which is then used by importers for clearing customs.
If you have any needs or questions, please contact us at service@hfoushi.com

