Over 10,000 cosmetics ingredients are in the trading market around the world. Only 8,783 are in the list of Inventory of Existing Cosmetics Ingredients in China (IECIC 2015). Cosmetics industry in Asia has a late start compared to the western countries, but now it is in the pace of fast development. The Transparency Market Research (TMR), a market research company from New York, has recently published a report of the Global Cosmetics Raw Materials Market. The data from the report shows that the market size of cosmetics raw materials worldwide reached 158.4 billion in CNY last year, and the size would become even larger, reaching 232.9 billion in CNY by 2025. The average growth rate of the global cosmetics raw materials market is predicted to be 4.6% from 2017 to 2025. In accordance with the market shares of different regions around the world in 2016, Asia-Pacific occupied the largest share of 32% of the global cosmetics raw materials market last year and China, Japan and India are the leading countries in Asia-Pacific.
According to the Regulations concerning the Hygiene Supervision over Cosmetics (1990), new cosmetics ingredients have to be ratified by the Ministry of Health under the State Council before they are used in the production of cosmetics. New cosmetics ingredients refer to the natural or artificial raw materials that are used to produce cosmetics for the first time in China. 10 new ingredients have been approved since 2004. (See Table 1)
Table 1, Approved New Cosmetics Ingredients 2004-2016
|
Ingredient Name |
Date of Ratification |
|
Alkyl(C12-22) Trimonium Chloride (Bromide) |
14.06.2004 |
|
Potassium Methoxysalicylate |
26.04.2007 |
|
9.69% Methylisothiazolinone |
28.05.2007 |
|
Carnitine (and) Tartaric Acid |
03.06.2008 |
|
Lathyrus Odoratus Flower Extract |
06.08.2008 |
|
Fructooligosaccharides |
06.08.2008 |
|
Polymethacryloyl Lysine |
19.03.2012 |
|
Dimethoxytolyl Propylresorcinol |
19.03.2012 |
|
Phenylethyl Resorcinol |
05.12.2012 |
|
Elaeagnusmollis Diel Oil |
30.10.2014 |
In September 2008, the competent authority for the hygiene administration of cosmetics was changed from Ministry of Health to China Food and Drug Administration (CFDA). In Table 1, the first 6 new ingredients in table 1 were approved by Ministry of Health, while the other 4 were ratified by CFDA.
Based on the data on the website of Health Food Review Centre of CFDA, about 181 applications were made between 2010 and 2016 (see Table 2) for the registration of new cosmetics ingredients. However, only 4 ingredients have been permitted to use in cosmetics.
Table 2, Summary of the Applications for Imported New Cosmetics Ingredients 2010-2016
|
Year |
First Time Application |
Supplement |
Re-check |
Total |
|
06. 2010 – 12.2010 |
20 |
3 |
0 |
23 |
|
2011 |
48 |
36 |
3 |
87 |
|
2012 |
40 |
22 |
3 |
65 |
|
2013 |
13 |
36 |
5 |
54 |
|
2014 |
11 |
44 |
1 |
56 |
|
2015 |
7 |
31 |
2 |
40 |
|
2016 |
13 |
31 |
0 |
44 |
|
Total |
152 |
203 |
14 |
369 |
According to Table 2, there are around 40 first time applications for imported new cosmetics ingredients per year on average from 2010 to 2012, yet the figure decreased to about 10 per year on average between 2013 and 2016. The years 2011 and 2012 witnessed the largest number of first time applications. This is because the Guidelines for Registration and Technical Review of New Cosmetics Ingredients was published and implemented on 1 July 2011. The release and implementation of the Guidelines indeed promoted the enthusiasm of applying for the registration of new cosmetics ingredients. However, the implementation of the Guidelines did not help increase the successful applications for the new ingredients used in cosmetics.
Table 3, Summary of the Applications for Domestic New Cosmetics Ingredients 2010-2016
|
Year |
First Time Application |
Supplement |
Re-check |
Total |
|
2010.6 - 2010.12 |
1 |
0 |
0 |
1 |
|
2011 |
5 |
4 |
0 |
9 |
|
2012 |
6 |
1 |
0 |
7 |
|
2013 |
5 |
3 |
0 |
8 |
|
2014 |
4 |
3 |
1 |
8 |
|
2015 |
5 |
3 |
1 |
9 |
|
2016 |
3 |
4 |
1 |
8 |
|
Total |
29 |
18 |
3 |
50 |
The number of applications for domestic new cosmetics ingredients is about 5 per year on average, which is much fewer compared to that of the imported new cosmetics ingredients. Elaeagnusmollis Diel Oil is the most recent domestic new ingredient that has been approved to be used in cosmetics.
Although the technical requirements for the registration of new cosmetics ingredients are lower than that of the registration of new drug, the registration period is almost the same. The reasons leading to the low passing rate are mainly reflected in the following aspects:
- Lack of unified reference list to determine whether an ingredient is regarded as new cosmetics ingredient at earlier stage.
- Imperfect management system e.g. the management of approval new cosmetics ingredients is not clearly indicated.
- Mandatory testing on animals is against the EU cosmetics regulation on full ban of animal testing since 2013.
- Actual technical review requirements are far more than that stated in the Guidelines for Registration and Technical Review of New Cosmetics Ingredients.
- No public window to discuss with internal review experts regarding technical issues directly .
- The necessity of technical innovation of cosmetics industry is less than that of drug and food industries .
Although only few new ingredients have been ratified since CFDA took over the competent authority of hygiene administration of cosmetics from Ministry of Health, several draft regulations have been published for public opinions by the government in order to manage the cosmetics ingredients market in a better and more scientific way. The progress of the management of cosmetics ingredients over the years are summarized as below:
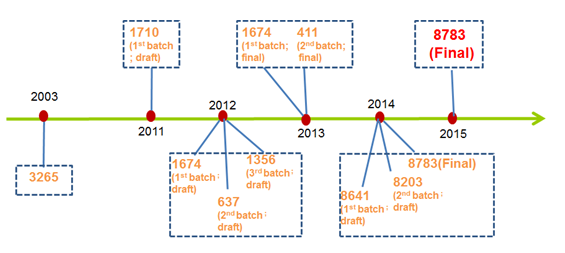
1. 2011 – 23.12.2015: Completion of the Positive List of Cosmetics Ingredients (Inventory of Existing Cosmetics Ingredients [IECIC])
2. 01.07.2011: The Implementation of the Guidelines for Registration and Technical Review of New Cosmetics Ingredients
3. 02.11.2011: The Notice on Regulating Related Matters of Administrative Permit of New Cosmetics Ingredients (Draft Version) Issued
- Before toxicology tests, examination of 3 batches of samples for the qualitative and quantitative methods of the raw material should be conducted.
- Providing methods of qualitative, quantitative and impurity test. If the methods are not subject to Hygienic Standard for Cosmetics (2007), national standard, industrial standard or any regulated documents issued by CFDA, complete testing methods should be submitted and validated by no less than 3 CFDA designated labs.
- Approval Notice to Approval License: From 1 April, 2014, Approval License of new cosmetics ingredients will replace Approval Notice. This license will be valid for 4 years and will be automatically abolished after the expiry date.
- Approval License can protect the rights of the applicant company for 4 years, while any approval new ingredients released via public notice can be manufactured, used, and marketed for every company: New approval ingredients can be manufactured, sold and used within the scope of the Approval License. Separate application should be made if companies need to use such new ingredients beyond the scope.
- Post-market surveillance to the applicant company: Complete system of retrospect and safety risk information collection should be established by the approval applicant. Regular updates and reports on the manufacture, marketing, usage and safety information should be made via web platform provided by CFDA
- Approval new in gre dients will be included in IECIC after 4 years of probation without any safety issues
20.07.2015: Regulations Concerning the Supervision and Administration of Cosmetics (Revised Draft Version) Issued
- IECIC list should be updated by the end of each year
- Registration is needed before use for high risks ingredients such as preservatives, hair dyes, sun-screening agents, colorants, skin whitening agents, etc. Record-keeping should be made for other new ingredients within 30 working days before use.
- For approved and recorded new ingr edients, applicant or domestic responsible agent should submit the reports of the use and safety information of the new ingredients every 6 months in 3 years.
6. 10.11.2015: Adjustment of Dossier Requirements for Registration of New Botanic Ingredients (Draft) Issued
- Redefining new botanic ingredients: refer to the natural raw materials from plants (including Algae) that are used to produce cosmetics for the first time in China , except single or highly purified substances extracted from plants.
- Adding exemption conditions of toxicology data: based on whether the ingredient is new in China or/and overseas, and if providing adequate proof to indicate the ingredient can be safely used in cosmetics or food for more than 5 years.
7. 10.11.2015: Guidelines of Cosmetics Safety Risk Assessment (Draft Version) Issued
From the series of regulations issued for the management of cosmetics ingredients between 2011 and 2015, it is speculated that cosmetics ingredients will be managed under classification depending on the degree of risk based on protecting the rights and interests of applicant. CIRS expects that the government will implement the previously issued regulations as soon as possible so that more new ingredients can be approved and used legally in Chinese market in the next 5 years. This will also help improve the technique of China’s cosmetics industry.
CIRS will hold the 3 rd Shanghai Summit Meeting on Cosmetic Regulations in Asia Pacific 2017 in Shanghai on 28 and 29 June, 2017, whereupon experts and government authorities from CFDA and other institutions will be invited to provide in-depth interpretation of supervisory regulations and future trends for the management of cosmetics ingredients in China (For more information of the Summit Meeting, please click here ).

