CIRS held a free webinar (download) on 26th and 27th Apr, 2018 on How to Comply with the Pilot Policy of Expanding 10 New Free Trade Zones (FTZs) for the Import of Non-special Use Cosmetics. The presentation includes 3 parts:
1) General info of pilot system in Shanghai Pudong new area and comparison with CFDA registration
2) Data analysis of record keeping in Pudong new area
3) Brief introduction and outlook of expanding 10 new free trade zones for the import of non-special use cosmetics.
Background
In order to implement the State’s decision making to unleash the vitality of companies’ innovation and entrepreneurship, and to promote a legalized, internationalized and facilitative business environment, the government decided to make a number of modifications on administrative licensing and decided to run it first in Pudong, Shanghai, and would expand to other cities if things went well.
On 17th Jan 2017, CFDA and AQSIQ jointly issued a “Notice on Pilot Implementation of Record-Keeping for Imported Non-Special Use Cosmetics in Pudong New Area” (Notice No. 7). On 18th Jan, CFDA issued another “Notice on Work Program of Record-Keeping for Imported Non-Special Use Cosmetics in Pudong New Area” (Temporary Notice No. 10) which gives clearer and more specific requirements. Later on 23rd Feb, CFDA issued an official “Clarification on the Work Program of Record-Keeping for Imported Non-Special Use Cosmetics in Pudong New Area” (Temporary). The Clarification explains the differences between the record-keeping system and registration with CFDA.
Scope
- First launched in Pudong, Shanghai
- Implementing time: 1st Mar 2017 – 21st Dec 2018
- Only for import non-special use products, first importation
- Importation can only happen at the corresponding port
- Overseas cosmetic company should have responsible person(s) registered in the corresponding area
- Cancellation of current record-keeping is required if the product is to be imported through other ports, re-do the record-keeping in another FTZ where the port is, or registration with CFDA is required
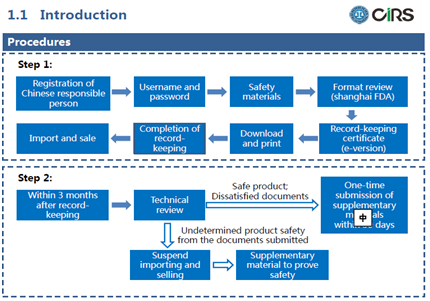
Procedures
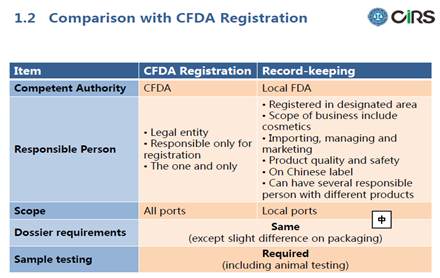
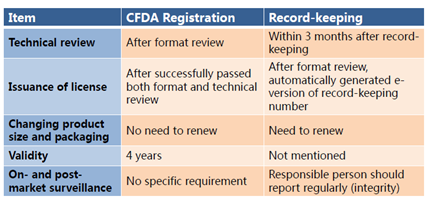
Comparison with CFDA Registration
Data Analysis
By the end of last month, there are a total of:
- 212 usernames
- 136 responsible persons
- 192 overseas manufacturers
- From 30 countries and regions, plus 20 domestic companies with their products produced outside China
We’ve summarized the data from Mar. 2017 to 20th Apr. 2018 and analyzed as below.
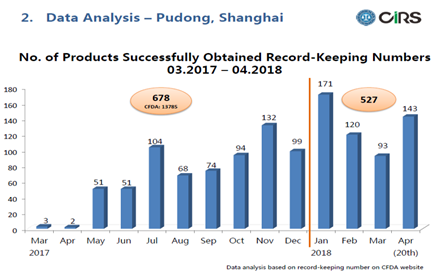
- 678 record-keeping numbers given out in 2017, and there are already over 520 numbers issued in 4 months time this year
- Compared to the total number of products registered with CFDA last year - 13,785, there is less than 5% of total import products recorded under the pilot system last year.
- We shall foresee an increase of this percentage this year as more companies are more familiar and have more confidence in the new system
In 2017, after a blank period of only 5 products obtained the record-keeping number in the first 2 months, there is a general growing trend of the numbers. At first, most of the companies trying this pilot system are the big companies.
|
Approval Passing Rate |
|||
|
Overall (from Mar 2017) |
~ 1/3 |
||
|
2018 |
1st |
2nd |
≥3rd |
|
56% |
29% |
15% |
|
- Overall passing rate – 1/3, which means there are over 3,000 products that have applied under this system
- The passing rate of this year increases, to over 50%, with no amendments needed. And about 85% of products have been approved after single time amendments, which makes the passing rate quite satisfying compared to last year
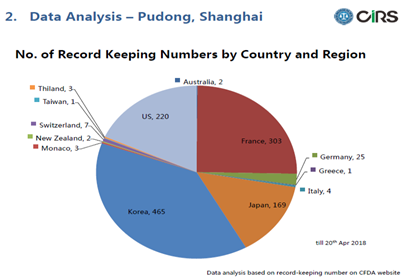
Here below is a pie chart of the No. of record keeping numbers by country and region. As we can see from the chart, Korea has the largest number, consists about 39% of the total. France and the US are the 2nd and 3rd, followed by Japan. The top 4 countries have covered 96% of the products recorded in Pudong.
Regarding countries, about half of the countries are from Europe.
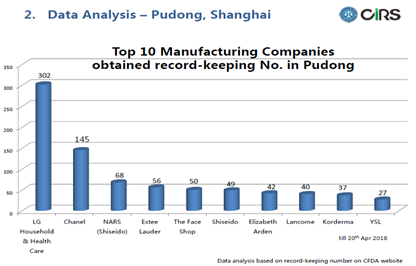
The bar chart below listed the top 10 manufacturing companies in regard to product numbers that have been recorded in Pudong. LG Household & Health Care is at the dominant position, doubles the number of products recorded by Chanel who is in the 2nd place. Nars from Shiseido and Shiseido itself have been separate listed, in the 3rd and 6th place respectively. Estee Lauder, The Face Shop, Elizabeth Arden, and Lancome have similar numbers, with Korderma and YSL in the 9th and 10th places. We can see that the top companies are mostly the well-known companies.
Typical problems especially for the pilot system
- Products should be cosmetics (used on healthy skin) and non-special use products
- Hair-dye: can be regarded as non-special use products if the color can be washed off immediately after dying
- Whitening products: physical whitening products, e.g. covering, are still considered as special use products
- Anything changes on packaging and Chinese label should be renewed as these are public information
Outlook of Expanding the Pilot Policy
After more than a year’s implementation of the pilot system, everything’s going smoothly according to Shanghai FDA, and the government thinks that the system can be copied and pasted to other FTZs. Therefore they issued a notice on 8th Mar 2018 to expand the pilot system to 10 other FTZs in China.
The 10 FTZs include Tianjin, Liaoning, Fujian, Henan, Hubei, Chongqing, Sichuan, Shaanxi, Guangdong, and Zhejiang. Among these 10 FTZs, Guangzhou and Zhejiang have already issued their notices to open the pilot system.
Zhejiang Zhoushan FTZ
- 120km2 implementing area
- Issued their notice on 6th Mar
- Held the press conference of launching the system, and the 1st record-keeping number of Zhejiang was issued
- Having the 2nd largest number of cosmetic factories in China
Guangzhou Nansha New Area FTZ
- 60km2 implementing area
- Issued notice on 11th Apr
- Implementation time: 8th Mar 2018 – 21st Dec 2018
- Having the largest number of cosmetic factories in China
To overseas manufacturers, the expansion of the system enables you to import through varies ports in different cities for different products, and you can have responsible persons in different cities. The market entrance permit in this way is less time-consuming and the process is easier.
The responsible person will take good responsibility for the products, including marketing activities and product safety. Competent authority will carry out their on-site supervision regularly. This results in a more stringent on- and post-market surveillance. Authorities will build-up an enterprise integrity system in the near future to grade the companies.
As FDA and CIQ have been combined to one department, they will have close contact and information exchange, this will make the process more efficient as well as having more rigid check on the information to avoid mismatch and result in misleading to our customers.
More details will be coming for these cities soon, and CFDA may issue further notice on what happens after the implementing time. We will keep updating the latest regulations. You can check on our website regularly or subscribe to our newsletter for any updates.
We are holding "The 4th Summit Meeting on Cosmetic Regulations in Asia-pacific" in June in Hangzhou. Officials and experts from CFDA and local FDA, and some experts from the US, EU and JP will talk about their regulations on our summit. You are very welcome to join us at the meeting!
If you have any needs or questions, please contact us at service@hfoushi.com.

