On September 21, 2020, the Cosmetics Supervision Department of the National Medical Products Administration issued the "Administrative Measures on Cosmetic Labeling" (draft for comments), and publicly solicited opinions from the public. Relevant opinions shall be fed back to huazhuangpinchu@163.com before October 20, 2020.
Cosmetic labeling is the most direct way for consumers to obtain basic product information and safe use methods. Before the adjustment of the functions of cosmetics regulatory agencies, cosmetics supervision and management have been implemented by multiple departments. All relevant departments that originally undertook the function of cosmetics supervision have issued related cosmetics labeling management regulations and documents. The current regulatory documents and national standards of cosmetics label management mainly include:
-
-
The departmental regulations "Administrative Regulations on Cosmetics Labeling" issued by the former AQSIQ (AQSIQ No. 100)
-
-
"Cosmetics Naming Regulations" and "Cosmetics Naming Guidelines" issued by the former State Food and Drug Administration
-
-
The mandatory national standard "Universal Labeling for Cosmetics" issued by the National Standards Committee (GB5296.3-2008)
However, due to the inconsistencies or conflicts in these regulatory documents and standards, the industry is at a loss. In order to facilitate the unified and standardized management of labels, the competent authorities have been drafting relevant management documents. For example, the former State Food and Drug Administration organized the drafting of "Prohibited Terms for Cosmetic Name Labeling" (draft for comments), which was solicited for external comments on June 26, 2009; it also issued the "Administrative Regulations on Cosmetics Labeling" (draft for comments) , soliciting opinions from the public on November 9, 2010. This regulation was again publicly solicited public opinions on April 29, 2011. The former Food and Drug Administration organized the drafting of the "Administrative Measures on Cosmetic Labeling" (Draft for Comment) (referred to as the "Measures"), and publicly solicited opinions in November 2014, and notified to WTO/TBT. However, due to the lack of a basis for higher-level laws, the former Food and Drug Administration temporarily suspended the legislative work of the Measures. Now because the new CSAR is determined, the State Administration for Market Regulation has also included the "Measures" in the legislative plan. The feedback collected during the public consultation process of the State Food and Drug Administration in 2014 was carefully sorted out, and the 2020 version of the draft for comment was formed based on the current market conditions and the management of cosmetics labels at home and abroad.
There are 34 articles in the Measures. Compared with the existing label management file, there are big changes. The main contents are summarized mainly from the following aspects:
-
-
Scope of application
Except the cosmetics that are only for export sales, it is applicable to the label management of cosmetics produced and operated in Mainland China, including cosmetics provided as the free gifts and trials in commercial activities.
-
-
The smallest sales unit of cosmetics should have a label [Minimum Sales Unit: For the purpose of product sales, it refers to the smallest packaging form when the product contents are delivered to consumers along with product packaging containers, packaging boxes, and product instructions. ]
-
-
The cosmetics registrant and recorder are responsible for the legality, completeness and authenticity of the cosmetics label
-
-
It is acceptable to stick the Chinese label for imported cosmetics, but the content of the product safety and efficacy claims should correspond to the relevant content of the original label
-
-
Labeling requirements for different types of products:
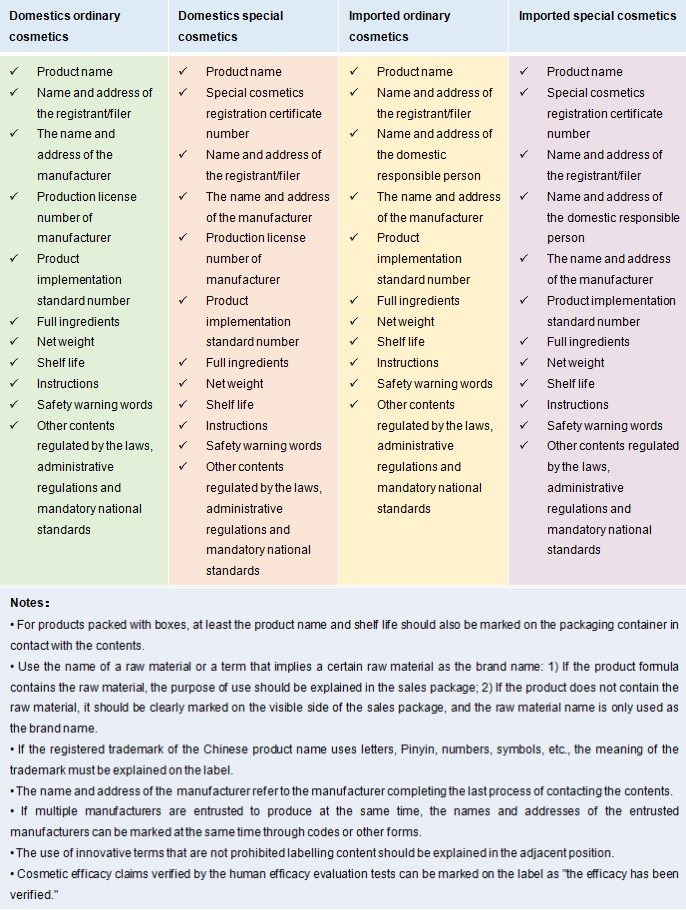
-
-
Brand name requirements
-
-
-
Comply with both the national trademark regulations and national cosmetics management regulations
-
-
Do not declare medical effects or the effects that the product does not have in the form of brand names
-
-
Use the name of a raw material or a term that implies a certain raw material as the brand name: 1) If the product formula contains the raw material, the purpose of use should be explained in the sales package; 2) If the product does not contain the raw material, it should be clearly marked on the visible side of the sales package, and the raw material name is only used as the brand name.
-
-
-
One prod uct, one number
The "Measures" no longer requires labelling filing number of ordinary cosmetics. But it is necessary to label the product implementation standard number. Except for cosmetics that do not require registration and filing, such as ordinary soap, the product implementation standards should be national or industry standards, and the registration or filing certificates for registered or filed cosmetics will contain the corresponding product implementation standard numbers. Product implementation standards include product name, product formula, production technology, sensory indicators, microbiological and physicochemical indicators and their inspection methods, use methods, storage conditions, shelf life and packaging materials, etc.
-
-
The trace components are clear at a glance
In addition to the requirements of maintaining the existing full-ingredient labeling and ranking in descending order of content for cosmetic ingredients, all ingredients in cosmetic formulations that do not exceed 0.1% (w/w) are classified as "other trace ingredients" and marked separately. According to the 6.4.3.2 of current GB 5296.3-2008 General Labeling for Cosmetics, when the added amount of ingredients is less than or equal to 1%, they can be arranged in any order. The new regulations on the labeling of cosmetic ingredients in the "Measures" can prevent merchants from exaggerating claims, and facilitate consumers to intuitively understand the micro-ingredients information in product formulations through labels, and to choose appropriate products combined with the information of efficacy claims on the label.
-
-
New labeling method of shelf life as "production date and use date after opening"
According to the current GB 5296.3-2008 General Labeling for Cosmetics, there are two labeling methods of shelf life mainly used: a) production date and shelf life; b) production batch number and expiration date. If the "production date and use date after opening" is adopted, the product shelf life should be no less than 36 months. In the European Union, when the product shelf life is not less than 30 months, it is necessary to mark the safe use time after opening.
-
-
Electronic labels are allowed on small size packages
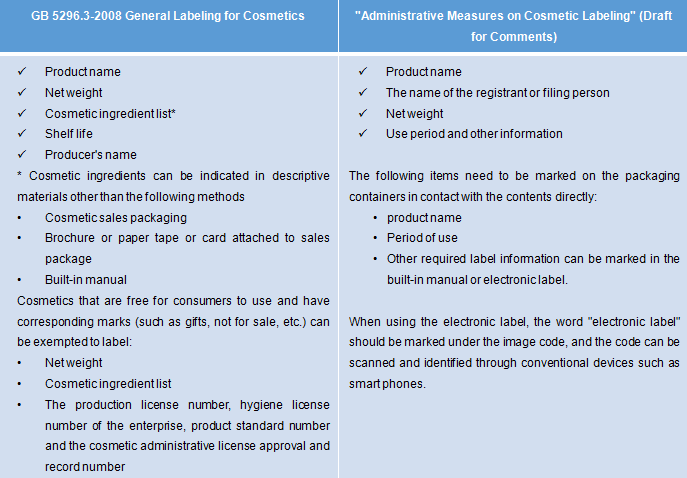
-
-
Efficacy claims are well-founded
The scientific basis for cosmetic efficacy claims includes relevant research data, efficacy evaluation data or literature data. The sources of research data include human tests, in vitro tests, consumer use tests, and other scientific research tests. The abstract of the scientific basis for the efficacy claims on the cosmetics label can be published on the website designated by NMPA for review and social supervision. The product efficacy is conducted through human efficacy evaluation test by an inspection agency with the corresponding qualifications for cosmetic safety and efficacy, and if the test results are deemed objective and effective, the efficacy "has been evaluated and verified" can be marked on the product label. For cosmetic efficacy claims that have not been verified by human efficacy evaluation tests, labeling the efficacy "has been evaluated and verified" on the product label will be regarded as an illegal act of "providing false materials." According to the evaluation principle table of efficacy claims, in addition to claims that prevent hair loss, anti-freckle and whitening, sunscreen, anti-acne (including blackheads removal), repair, tear-free formula and specific new efficacy request human trial, other efficacy claims can be evaluated by another efficacy evaluation methods. However, if the company wants to legally mark a certain efficacy effect "has been evaluated and verified" on the packaging as the product promotion content, the company will be more willing to choose the human trail method.
Table 1 Evaluation principle of efficacy claims
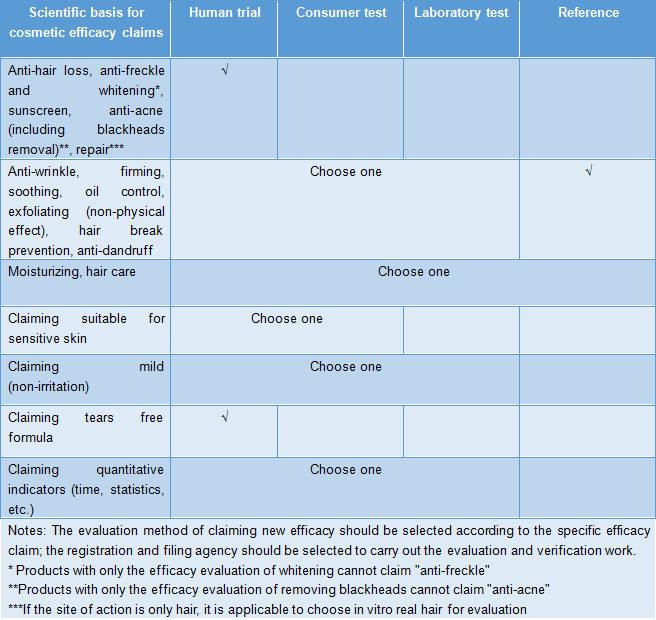
-
-
Thirteen items prohibited to be marked or declared
There are 13 items forbidden to label or claim in the "Measures". Compared with the 8 prohibited labeling items in the Cosmetics Naming Regulations, the "Measures" detailed its content, and the new content mainly includes:
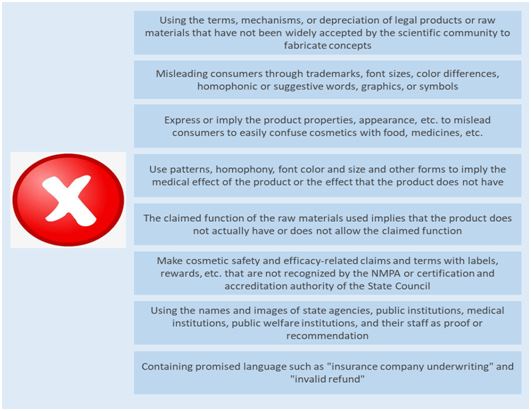
-
-
Implement dynamic management of prohibited words
The banned words of cosmetics labelling claims are subject to dynamic management. NMPA will make real-time adjustment to the banned words based on the actual cosmetics supervision work. There is no list of banned words in the draft for comments. According to the 2014 version of the "Administrative Measures on Cosmetics Labeling", the list of banned words for cosmetics labeling mainly includes:
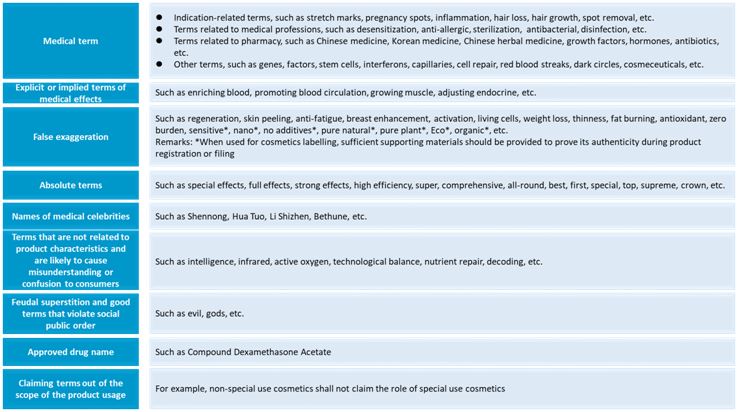
-
-
Legal responsibility
1) If the cosmetics labeling content is inconsistent with the relevant content of the product registration or filing, the cosmetics are subject to a situation as" the cosmetics production and operation do not meet the mandatory national standards, technical specifications, or technical requirements specified in the cosmetics registration and filing materials":
-
-
Confiscation of illegal income, illegal production and operation of cosmetics, and raw materials, packaging materials, tools, equipment and other items specially used for illegal production and operation; if the value of illegal production and operation of cosmetics is less than 10,000 yuan, the penalty shall be more than 10,000 yuan and less than 50,000 yuan;
-
-
If the value of the goods is more than 10,000 yuan, a fine of more than 5 times and less than 20 times the value of the goods shall be imposed;
-
-
If the circumstances are serious, it shall be ordered to suspend the production and business, the filing info shall be canceled, or the cosmetics registration license shall be revoked, and the legal representative or main responsible person of the illegal unit, the directly responsible supervisor and other directly responsible persons shall be fined of 1 to 3 times the income from the unit in the previous year, and prohibited from engaging in cosmetics production and business activities within 10 years;
-
-
If a crime is constituted, criminal responsibility shall be investigated according to law
2) The cosmetics are subject to a situation as “the production and operation label do not meet the requirements of these regulations":
A. The content and form of the label do not meet the requirements of cosmetics
B. Labeling or claiming prohibited content
C. If the name of other enterprises or institutions is marked with "joint research and development", "producer", etc. as the introductory language, after verification the marked enterprise or institution is unknowing [Penalizing the registrant and the filing person] , Or the marked enterprise or institution has informed consent, but not actually participating in the relevant production and business activities [Penalizing the registrant, the filing person and the marked enterprise or institution]
-
-
Confiscation of illegal income, illegal production and operation of cosmetics, and raw materials, packaging materials, tools, equipment and other items specially used for illegal production and operation;
-
-
if the value of illegal production and operation of cosmetics is less than 10,000 yuan, the penalty shall be more than 10,000 yuan and less than 30,000 yuan;
-
-
If the value of the goods is more than 10,000 yuan, a fine of more than 3 times and less than 10 times the value of the goods shall be imposed;
-
-
If the circumstances are serious, it shall be ordered to suspend the production and business, the filing info shall be canceled, or the cosmetics registration license shall be revoked, and the legal representative or main responsible person of the illegal unit, the directly responsible supervisor and other directly responsible persons shall be fined of 1 to 2 times the income from the unit in the previous year, and prohibited from engaging in cosmetics production and business activities within 5 years;
-
-
If the cosmetics labels have defects but do not affect the quality and safety and do not mislead consumers, they shall be ordered to make corrections; if they refuse to make corrections, they shall be fined less than 2,000 yuan.
3) In the case of "providing false materials":
A. Falsify the scientific basis of efficacy claims, or claim the efficacy of cosmetics that have been verified on the label without performing human efficacy evaluation tests.
B. The relevant information of the registrant, recorder, or manufacturer marked on the label is untrue, forging or fraudulently using other factory’s name and address and other info.
Special cosmetics:
-
-
Refusal to approval
-
-
The administrative license shall be revoked for registered products, it is not allowed to apply for the cosmetics registration within 5 years, and the illegal income and cosmetics that have been produced and imported will be confiscated;
-
-
If the value of cosmetics that have been produced or imported is less than 10,000 yuan, a fine of 50,000 yuan up to 150,000 yuan will be imposed;
-
-
If the value of the goods is more than 10,000 yuan, a fine of 15 times to 30 times the value of the goods shall be imposed;
-
-
The legal representative or main responsible person of the illegal unit, the directly responsible supervisor and other directly responsible persons shall be fined of 3 to 5 times the income from the unit in the previous year, and prohibited from engaging in cosmetics production and business activities for life.
Ordinary cosmetics:
-
-
The filing info shall be canceled, and it is not allowed to apply for the cosmetics filing within 3 years, and the illegal income and cosmetics that have been produced and imported will be confiscated;
-
-
If the value of cosmetics that have been produced or imported is less than 10,000 yuan, a fine of 10,000 yuan up to 30,000 yuan will be imposed;
-
-
If the value of the goods is more than 10,000 yuan, a fine of more than 3 times and less than 10 times the value of the goods shall be imposed;
-
-
If the circumstances are serious, it shall be ordered to suspend production and business until the cosmetics production license is revoked, and the legal representative or main responsible person of the illegal unit, the directly responsible supervisor and other directly responsible persons shall be fined of 1 to2 times the income from the unit in the previous year, and prohibited from engaging in cosmetics production and business activities within 5 years.
4) In the case of "failure to publish a summary of the basis for cosmetic efficacy claims in accordance with the provisions of this Regulation":
A. Not publish the summary of the basis for cosmetic efficacy claims
B. The content of the published summary does not meet the requirements of the Measures
-
-
Order to make corrections, give warnings, and impose a fine of 10,000 yuan up to 30,000 yuan;
-
-
If the circumstances are serious, order to suspend production and business, and impose a fine of 30,000 yuan up to 50,000 yuan, and the legal representative or main responsible person of the illegal unit, the directly responsible supervisor and other directly responsible persons shall be fined of 10,000 yuan up to 30,000 yuan.
Compared with the 2014 version of the "Administrative Measures on Cosmetics Labeling" (draft for comments), imported cosmetics are allowed to be labeled in Chinese, but the content of product safety and efficacy claims should correspond to the original label. Due to the different international management requirements and standards for the safety and efficacy claims of cosmetics, this regulation will greatly increase the possibility of imported cosmetics companies to provide commercial packaging designed specifically for the Chinese market, which will increase the company's operating costs and cycle. Regarding the requirements for the terms of efficacy claims, please refer to the list of banned terms for cosmetics label claims to be released soon. The “Measures” clarify the corresponding legal responsibilities in cases where the content of cosmetics labels is inconsistent with the relevant content of product registration or filing, the labeling and claiming prohibited contents, the falsification of scientific basis for efficacy claims, and the unpublished summary of the basis of cosmetic efficacy claims, in serious cases, the company and the person in charge will face a fine of 30 times the value of the goods and a lifetime ban from engaging in cosmetics production and business activities. Consumers mainly obtain product information through two channels when purchasing cosmetics: one is the label information on the product packaging; the other is product information transmitted through other media such as shopping guides, paper or electronic promotional materials, and TV advertisements, etc. The 2020 version of the "Administrative Measures on Cosmetics Labeling" (draft for comments) contains 13 prohibited labeling or claiming contents that comprehensively summarize the common publicity methods of cosmetics currently placed on the market. After confirmation, the “Measures” apply to all cosmetics sold online and offline in China (except for cross-border e-commerce). The implementation of the "Measures" means that in addition to cosmetics sold through cross-border e-commerce, the claims of cosmetics sold through other channels will be comprehensively supervised, rectified and purified, making the wide variety of cosmetic claims clearer and easier to understand. It is beneficial to instruct the consumers to choose their required products scientifically.
If you have any needs or questions, please contact us at service@hfoushi.com.

